Wanted: ROI-Driven MedTech Startups and Better Access to Telehealth (MedTech Download)

Key Takeaways
- Medtech startups rejoice – health systems are still investing with the right companies: So what do these “right” companies have in common? A clear and demonstrative pathway to ROI.
- Don’t call it a comeback: The adoption of telehealth has been growing for years, but in the wake of the pandemic, access to these technologies has, in many cases, become a necessary point of care.
- There shouldn’t be surprises when it comes to FDA approval: A critical component of bringing a medical device to market lies in knowing – and benchmarking – FDA clearance requirements well before submission.
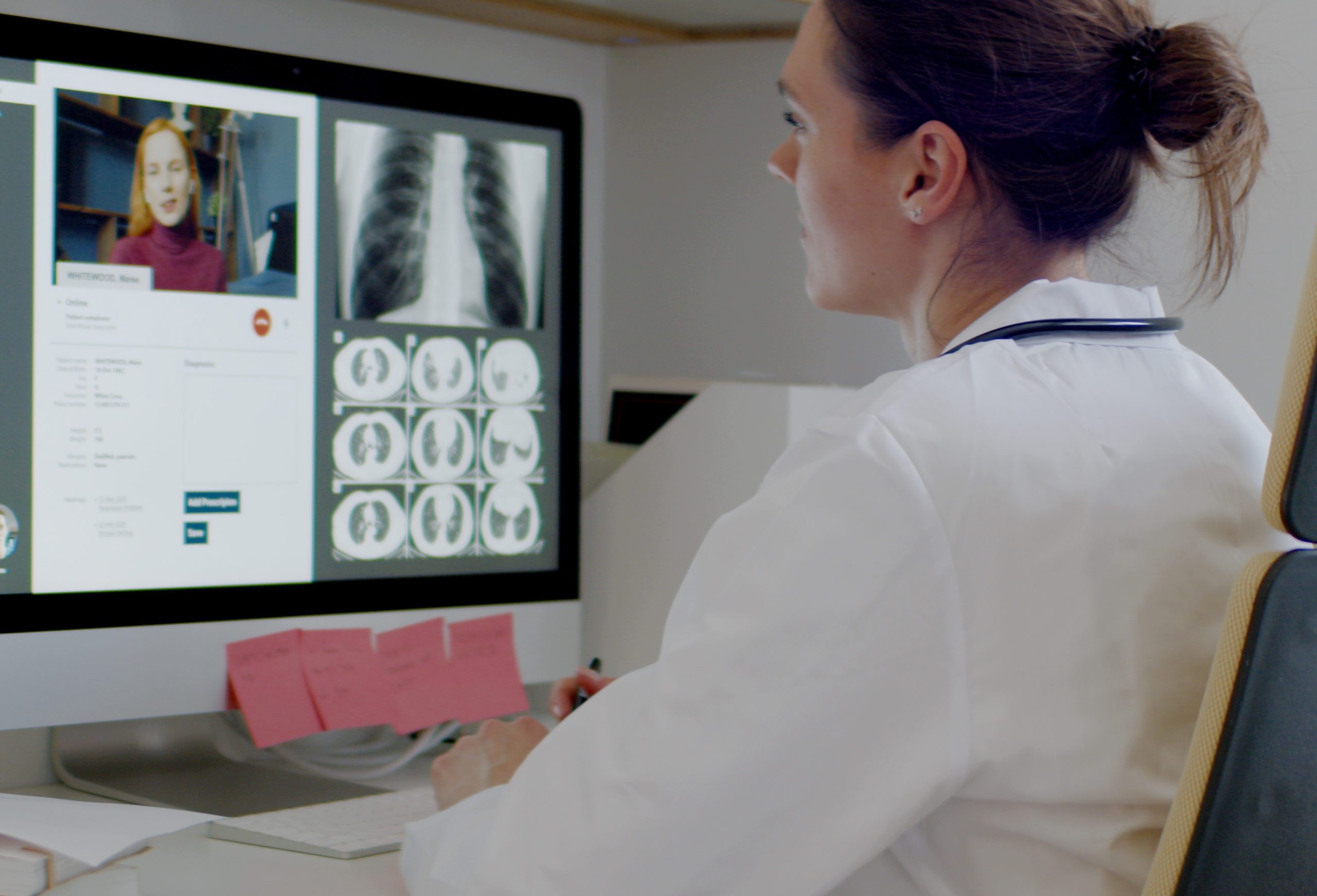
The Ripple of Telehealth Adoption During COVID-19

For the past seven months, we have seen our world change in ways we could have never predicted. Navigating a public health crisis is a challenge in any industry, but for the medical community, adjusting to life with the virus has carried additional complexities related to patient equity and access to care. One tool that has come to the forefront is telemedicine, however these technologies certainly are not new.
Industry innovators like eVisit have pioneered this new way of thinking and provided doctors the ability to treat patients remotely for several years. But prior to 2020, adoption of these platforms had been somewhat limited. In this article from Healthcare Dive, the AMA explores the surge in telehealth as a way forward for the medical community during the pandemic – and well beyond.
Neovasc Angina Device Fails to Win FDA Panel Backing, Stock Tumbles 43%

When assessing product viability, there is little doubt that what you don’t know can indeed hurt you. Canadian company Neovasc learned this lesson in a jaw-dropping announcement that caused it’s stock to lose nearly half its value. Neovasc CEO would probably love to lay the blame for this shocking turn of events on #2020. However, the outcome of the FDAs decision to deny approval of its angina device was not due to bad luck.
When it comes to planning for FDA clearance, foresight is everything. The device success process starts long before seeking approval – and finishes long after. Too often we have seen this scenario – companies trying to convince a panel of clinicians that benefits outweigh the risks or their device is an improvement on the current standard of care. And in many of those cases, just like Neovasc, they fall flat. The reality is that clinical evidence benchmarks must be in place early on. And when companies live and breathe these metrics, FDA decisions aren’t a surprise.
Healthcare CIO Survey Reveals How Covid-19 is Impacting Startup Collaboration

The healthcare industry’s response to COVID-19 felt at times like a tale of two cities. On one side, hospitals were overwhelmed. Physicians were under-resourced and taxed, desperately seeking innovations in medicine and medical technology to aid in treating patients and stopping the spread of the virus. On the other side, elective procedures and other treatments deemed non-essential were delayed or cancelled entirely, leaving some medical institutions with billions of dollars in lost revenue.
Now those losses, as well as others associated with COVID-19, are impacting decisions related to how health systems approach their relationships with medical startups. But the news isn’t all bad. While in many cases budgets are reduced, a survey by Dreamit Ventures and MedCityNews revealed that leaders are still looking to invest, but only in startups “that can support a clear and immediate ROI.” Todd Dunn, vice president of innovation and innovator-in-residence at Atrium, reiterated this, stating that “[It’s] just surprising how many startups present ROI stories with minimal data that either lack credibility or are simply based on weak or unproven assumptions. Feels like the ROI slide is often just a placeholder in their deck.” The clear lesson? Upstream marketing paired with a proven, ROI-driven go-to-market strategy is your lifeline.
Flexible Skin Sensor to Help ALS Patients Communicate
Stephen Hawking was one of the intellectual marvels of the world, accomplishing incredible scientific feats in what should have been a short life. But in longevity he was just as exceptional, defying the odds and living well beyond what doctors expected when he was initially diagnosed with ALS at the age of 21. At that time he was told he had only a short span to live, but miraculously he survived another 56 years.
Over those same decades, the technology that we use to treat people affected by Hawking’s disease has transformed lives, with many of them aimed at ensuring ALS patients can engage with the world as richly and completely as possible. Researchers at MIT recently announced the development of a new sensor patch that allows ALS patients to communicate through small movements of the face. The technology is both cheaper and may be more effective than its counterparts that currently exist in the market.
Want an easy way to stay up-to-date on everything that matters to medtech entrepreneurs and executives? Subscribe below to receive industry insights, medtech resources, and more, delivered directly to your inbox.