This is What’s Needed to Truly Pivot to Digital Healthcare (The MedTech Download)

Key Takeaways
- To promote digital health’s success in the long-term, it is critical to put infrastructure in place that ensures sustainability for patients, providers, and other stakeholders.
- AI and digital health technologies dominated the most recent batch of Breakthrough Device Designations.
- While most U.S. healthcare consumers plan to return to in-person doctor visits, 42% are either showing hesitancy or are outright refusing, despite the increase in vaccinations.
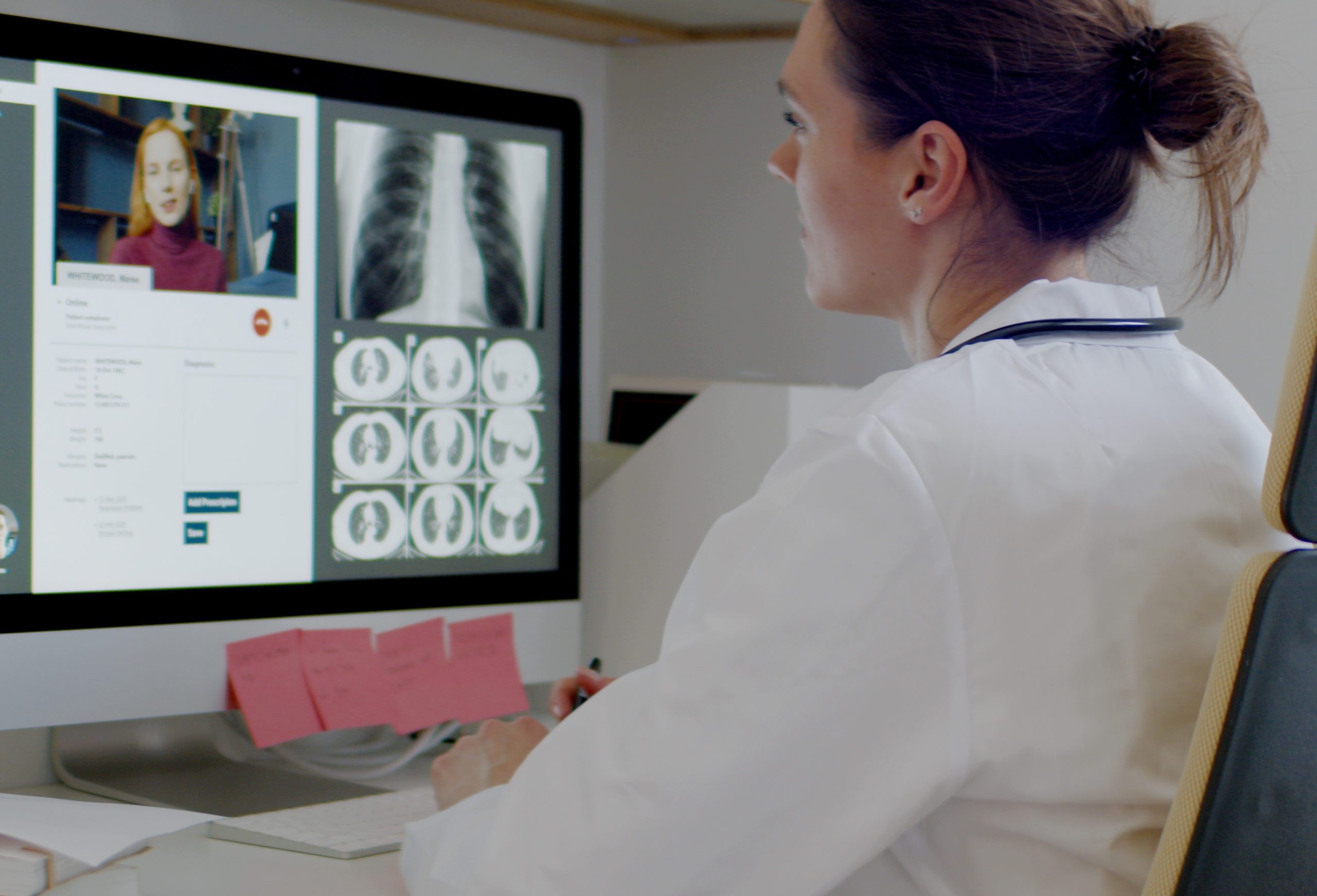
The Transformation to True Digital Healthcare

The story of the U.S. healthcare system is not one of a coordinated plan. In large part, the system we navigate today was built like a layer cake, each piece hastily placed atop another, with little consideration of how they would fit together. First, physicians and hospitals began to organize, with the rise of the AMA in the early 1900s. Then, during the Depression, we witnessed the infancy of our current payer system. Employers would contract with hospitals for “prepaid service plans,” which, according to the AMA, was more about profit than patients. According to the organization’s Journal of Ethics, “while they gave consumers an affordable way to pay for inpatient care, their primary purpose was to assure hospitals a steady income stream during a period of declining revenues.” Flash forward to today, and many pains of U.S. healthcare have their roots in the unintentional nature of the way we built our system.
When it comes to the recent rise of digital health, many argue that we have an opportunity to get it right this time when it comes to building a sustainable, effective system. As an article in MedTech Intelligence argues, “COVID-19 may have catalyzed the industry’s shift to digitally-enabled care, but many of the solutions and delivery channels created were not built with sustainability and longer-term impact in mind.” The article goes on to discuss the legal, regulatory, and infrastructural elements needed to successfully implement digital health in the long-term.
Survey: 42% of Americans May Not Go Back to In-Person Care this Year

In 2020, COVID-19 dominated the headlines and the healthcare industry. We tallied case counts, hospitalizations and deaths each day, with many people choosing to shelter in place to avoid infection. It was at this time that many Americans who needed care made a choice. With in-person doctor visits off the table, Americans either embraced telemedicine or delayed care. For those in the latter group, reports are now surfacing that this choice is having dire consequences. A recent study of radiation oncologists is reporting an “increase in new patients with advanced-stage cancers coming into clinics for treatment, compared to before the pandemic.”
Now, in 2021, the good news is that, as more people become vaccinated, more than half of Americans plan on returning to their physicians’ offices. The bad news? Despite the shift, “20% of U.S. healthcare consumers are still planning to delay physician visits this year and 22% are unsure, according to a new survey from patient access solutions provider Kyruus.” The survey also revealed that, while in-person visits are preferred, 30% of respondents are more likely to choose virtual as a result of the pandemic. For more stats, click here.
AI, Digital Health Feature in Latest Batch of FDA Breakthrough Device Designations

Waiting on FDA approval is a hurdle that almost all new medical devices must clear. And while the time it takes to get a nod from the agency can vary, there are cases in which expediting the process is in the public interest, especially in the case of life-threatening or irreversibly debilitating diseases or conditions. It is for this reason that the FDA announced the Breakthrough Devices Program, which replaced the Expedited Access Pathway and Priority Review for medical devices. This voluntary program for certain medical devices and device-led combination products seeks to “provide patients and health care providers with timely access to these medical devices by speeding up their development, assessment, and review, while preserving the statutory standards for premarket approval, 510(k) clearance, and De Novo marketing authorization, consistent with the Agency’s mission to protect and promote public health.”
As reported in MedTech Dive, the most recent recipients of this designation are a batch of AI and digital health companies. This includes Koios Medical, whose AI software enables the early detection of breast and thyroid cancer. Also on the list is Nēsos,’ a prescription digital therapeutic for rheumatoid arthritis. The device “consists of earbuds that deliver electrical signals to modulate neurological pathways and, in doing so, rewire the immune system to reduce the severity of rheumatoid arthritis.” The article goes on to list other Breakthrough Device Designations for companies like Implandata Ophthalmic Products, Medtronic, and more.
FDA Shares Plan for Restarting Facility Inspections, Warns Backlog Will Persist into 2022

Last year was a challenging one for the FDA. In the wake of the COVID-19 pandemic, the agency chose to pause most foreign and domestic inspections except mission-critical inspectional work, due to federal guidelines to mitigate the spread of the virus. Soon after, the agency began to use alternative methods, including virtual inspections, to progress its work as much as possible. In July of 2020, after establishing a COVID-19 advisory system, more inspections took place. Yet the agency acknowledges that, even with these measures in-place, the FDA is experiencing a significant backlog, one that will continue into 2022.
Recently the FDA released a roadmap that addresses the backlog and it’s plan to navigate through it. As reported, “The device division will prioritize application approvals for high-priority products and other inspections deemed to be mission critical. Post-approval inspections and routine surveillance are the lowest priority activities.” In the best case scenario, the agency hopes to resume normal operations by July of next year.
Stay up-to-date on everything that matters to MedTech entrepreneurs and executives. Subscribe below to receive industry insights, MedTech resources, and more delivered directly to your inbox.