How Should Software as a Medical Device be Evaluated in the MedTech Industry? (The MedTech Download)

Key Takeaways
- As technology in healthcare has grown, the need to validate software as a medical device, or SaMD, is more critical than ever.
- Last year’s mass adoption of telemedicine ensured people still had access to medical care in the wake of the pandemic. But now some physicians and patients are experiencing growing pains as the technology becomes a permanent part of their workflow.
- Measuring blood pressure via a wearable device, such as a smartwatch, could have immense benefits for those struggling with hypertension. Now Fitbit is conducting a trial to see if its technology can do so accurately and independently.
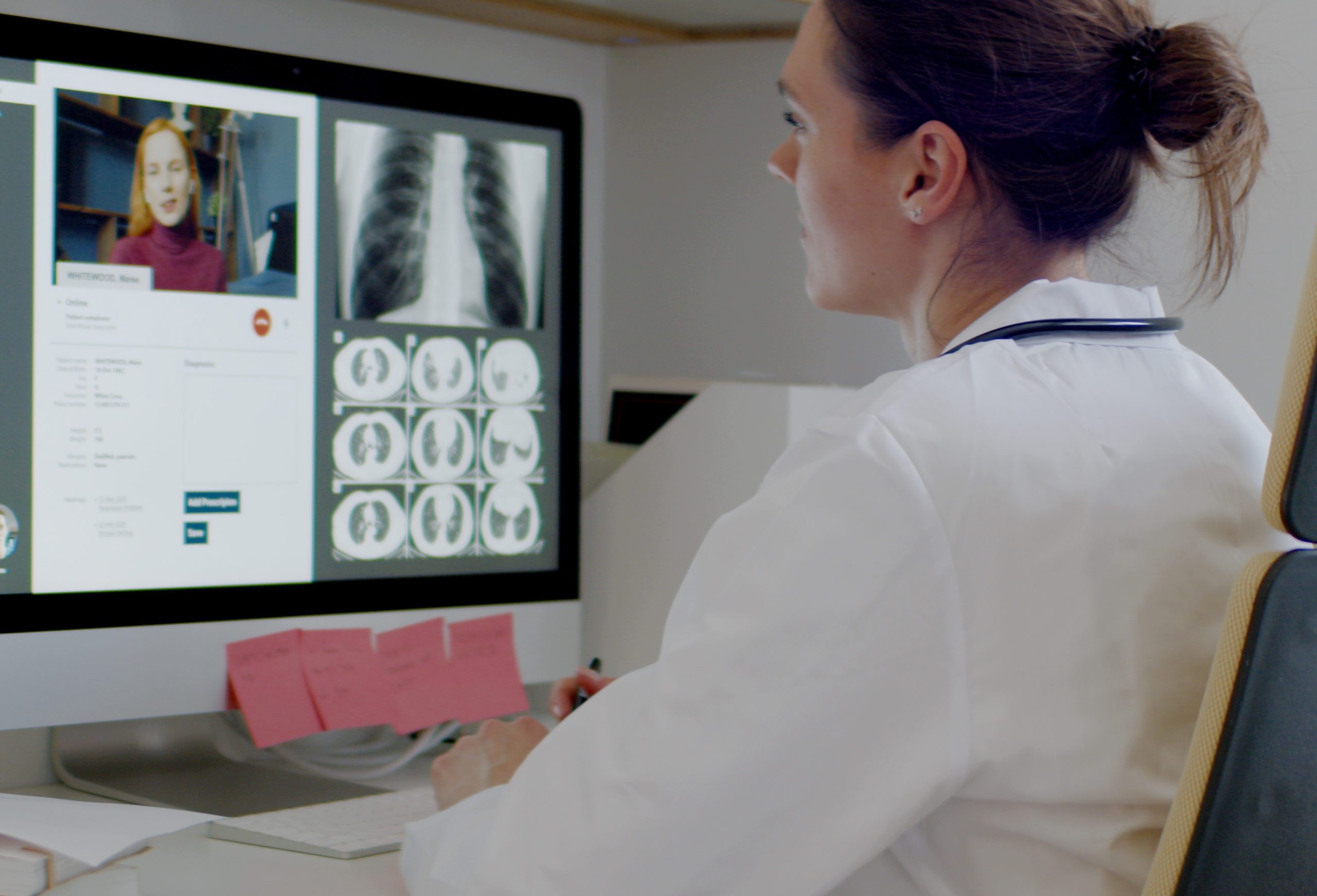
Validating Software as a Medical Device (SaMD)

During the rise of digital health and telemedicine over the past 2 years, discussions surrounding technology in the healthcare system are at the forefront. Many experts have cited the industry’s historical hesitance to adopt technologies such as AI and machine learning. But now hesitancy seems to be yielding to the reality that these innovations are expanding access to, and increasing the quality of, healthcare.
And often technology in healthcare has not only supplemented medical devices, but become the device itself. This has paved the way for SaMD, or software as a medical device. An article in Medical Device + Diagnostic Industry explores this concept, and how to properly validate SaMD to ensure “compliance, patient safety, and product quality.”
The Pandemic Made Telemedicine an Instant Hit. Patients and Providers Feel the Growing Pains.

When it became clear that the pandemic would require us to adjust to a locked-down world, those in healthcare saw telemedicine in a new light. Contrary to what much of the public may believe, in 2020, telemedicine was nothing new. Far from it. For years, telehealth companies extolled the virtues of their product: expanded access to care, an increase in the number of patients doctors could see per day, and more. But the healthcare community was slow to adopt this new way of working. Until the pandemic forced them to reconsider.
Now telemedicine, digital health, software as a medical device, and acnilary technologies have asserted themselves into the mainstream. But that doesn’t mean that there haven’t been challenges along the way. An article published by Kaiser Health News explores the many road bumps that telemedicine has presented to providers and patients, from connectivity to customer service.
Oscillating Magnetic Field Shrinks Glioblastoma Tumor

A cancer diagnosis can be devastating for patients, both in prognosis as well as the invasiveness of current treatments. Radiation, chemotherapy, and surgery can be painful, dangerous, and disruptive to quality of life, adding another layer of burden to an already stressful diagnosis. And in some aggressive cancers, such as glioblastoma, even these approaches may not result in remission.
Recently researchers at Houston Methodist Neurological Institute developed a device that succeeded in shrinking a glioblastoma tumor in a patient volunteer. It used “an oscillating magnetic field to disrupt biochemical processes in cancer cells,” which is thought to disrupt “electron transport during mitochondrial respiration, leading to an increase in reactive oxygen species and eventual cancer cell death.” The results of the trial opens the door to the potential for a future non-invasive and non-toxic brain cancer treatment.
Will Wearables Ever Accurately Measure Blood Pressure?

Wearable technology has grown in leaps and bounds over the past decade, and with that growth, applications for the devices have expanded as well. Currently your Fitbit or Apple Watch can track your heart rate and activity level; it reminds you to stand up each hour. But that is just the beginning, as technology companies continue to seek out new functionality to help users access a wider variety of health metrics.
But one key health issue that affects millions of Americans each year is blood pressure. As Medical Device Network points out, “every year, 9.4 million people die from complications related to high blood pressure, according to the World Health Organization.” The only way to properly monitor hypertension is consistent blood pressure monitoring, but there are challenges related to at-home solutions. The article goes on to explore Fitbit’s recent announcement that it is conducting a trial to see if it’s smartwatches can accurately measure blood pressure, which would provide patients with a welcome tool to improve their health. However, the post also reports that doctors still may need some convincing related to relying on data from wearables.
Stay up-to-date on everything that matters to MedTech entrepreneurs and executives, from opportunities within software as a medical device to fundraising. Subscribe below to receive industry insights, MedTech resources, and more delivered directly to your inbox.