As the Virus Surges, GE Healthcare Pioneers “Virtual ICU” Technology (MedTech Download)

Key Takeaways
- GE Healthcare’s Virtual ICU connects intensivists with ICU patients in rural areas across Oregon. The platform helps to support doctors in the field who are fighting COVID-19 in their communities.
- To reduce medical waste and associated emissions, experts are making the case for medical device reprocessing over single-use products.
- In response to a request from the FDA, medical device company Penumbra recalled its Jet 7 Xtra Flex catheter device due to the risk of injuries – and even death – while removing clots in stroke patients.
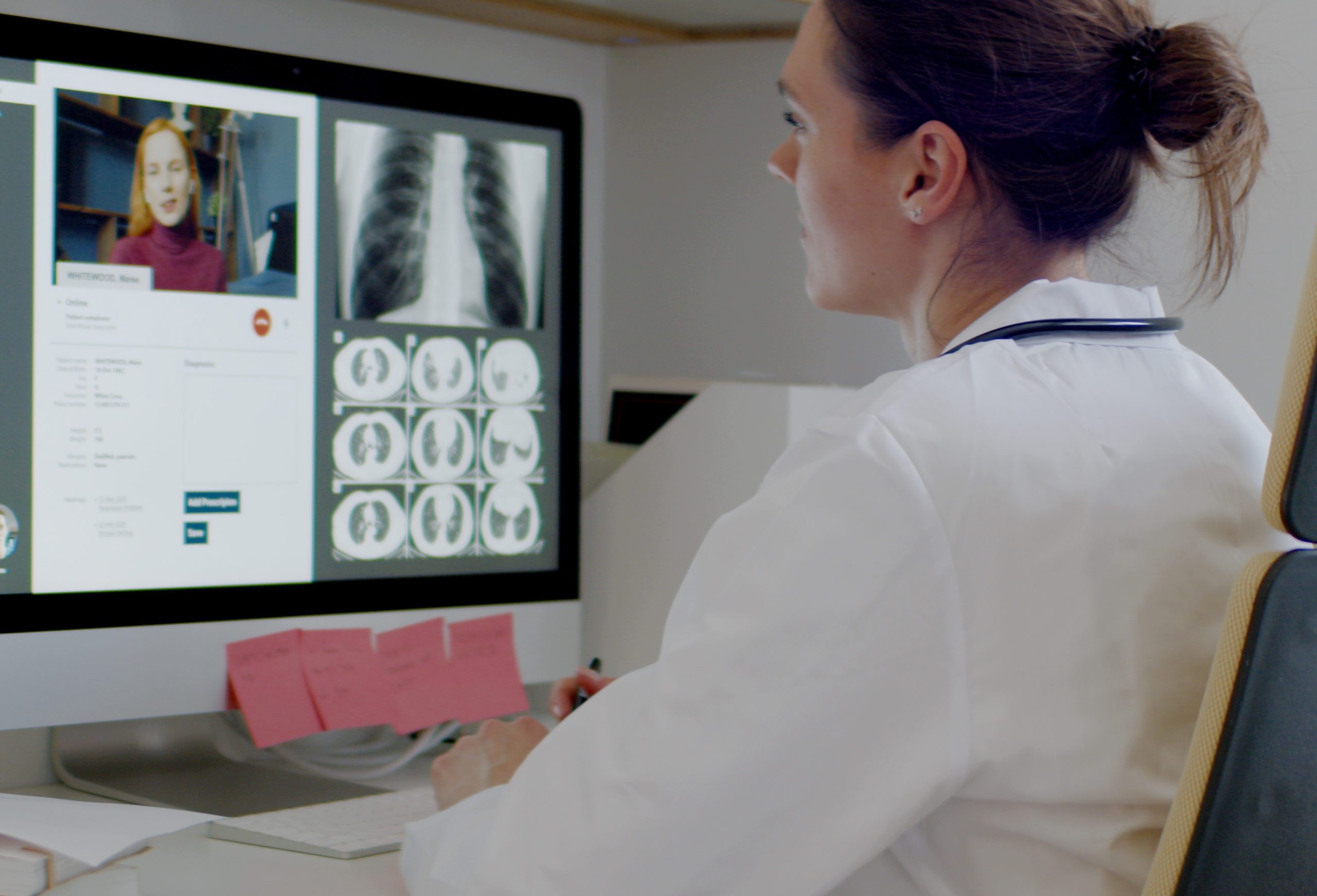
GE Healthcare Helps Set Up a ‘Virtual ICU’ Across Oregon

While many hoped that the worst of the pandemic was behind us after this spring, the winter has proven to be as challenging as experts predicted. Infections and deaths are at an all-time high and projected to grow exponentially after the holidays. With this naturally comes concerns related to hospital resources, as ICU beds become scarce and hospitals are stretched beyond capacity.
For hospitals in rural areas with even more limited resources, these challenges are amplified. To combat this problem, GE Healthcare announced a multi-year agreement with Oregon Health & Science University (OHSU) to virtually connect intensivists with ICU patients across the state. As reported in MassDevice, according to Joe Ness, OHSU Healthcare’s SVP and COO, “the VICU allows us to execute our vision of increasing the level of care in community hospitals, allowing patients across the state of Oregon to receive the care they need closer to home while reducing unnecessary transfers and optimizing ICU capacity in the area,” Ness said.
Should Hospitals Reuse Medical Supplies? A New Study Says Yes

At the beginning of the pandemic, healthcare systems were scrambling to respond to the unprecedented influx of patients coming into hospitals and ICUs. And, almost immediately, institutions sounded the alarm related to supplies and medical equipment. Access to ventilators became critical, with companies like Dyson jumping in to provide more machines. Just as important was the need for PPE, as doctors and nurses reported troubling shortages, many of them falling ill themselves.
In a convergence of both necessity and conservation, the concept of medical device reprocessing has become a topic that is timely on both a micro and macro level. Salon recently reported on the many benefits of medical device reprocessing, which would “cut down on medical waste and the emissions produced by manufacturing new medical supplies.” Read the full article here.
At FDA’s request, Penumbra Recalls Catheter Device After 14 Patient Deaths

In the medical device world, receiving FDA approval and clearance is a critical part of bringing an innovation to market. Proving safety and efficacy is an often years-long, expensive, painstaking process, which is why the word “recall” is one that every MedTech executive is loath to imagine, much less hear. Depending on the stage of the company, a recall can be either an expensive setback or the end of the entity altogether.
Medical device company Penumbra recently recalled its Jet 7 Xtra Flex catheter device due to the risk of injuries and even death while removing clots in stroke patients, following advice from FDA. And while the company sits firmly in the “this won’t end us” category, the recall results not from a technique issue, but a serious problem with the product’s distal tip. MedTech Dive reported that the tip “was susceptible to damage while in use, which created the risk to patients during pressurization or contrast injection, according to the company.” While this product is specialized, the situation is food for thought for any company bringing a product to market.
Philips Puts Down $2.8B for BioTelemetry and Its Wearable Heart Monitors

Over the past few months, the migration of healthcare to a remote environment has been unprecedented, much like its catalyst. The doubling down on telehealth has been in stark contrast with “before,” when many telehealth companies experienced a sluggish reception related to broad adoption. But not anymore. According to a recent survey, “close to half of all physicians (48%) are treating patients through telemedicine, up from 18% in 2018.” And even now that we have a vaccine, norms related to healthcare have changed, and telehealth is expected to continue to experience growth in the years to come.
And with this paradigm change, some of the largest companies in the world are reacting. Fierce Biotech reported that Philips signed a $2.8 billion deal to acquire BioTelemetry to “broaden the reach of its digital patient monitoring platforms, from inside the hospital out to the home.” Currently BioTelemetry “helps remotely monitor and diagnose at least 1 million cardiac patients each year.” Philips expects to grow BioTelemetry’s business by 20% over the next 4 years, anticipating what can only – and repeatedly – be referred to as our “new normal.”
Stay up-to-date on everything that matters to MedTech entrepreneurs and executives. Subscribe below to receive industry insights, MedTech resources, and more delivered directly to your inbox.